This article is a translation by MSC’s Rolf Schonfeld from the original German “Schneekristalle – Juwelen des Himmels” by Dr. Norbert Span.
Snowflakes are just ice crystals stuck together. The recipe is very simple: One oxygen atom and two hydrogen atoms, add cold temperatures and you get ice. This seemingly trivial structure however is full of surprises, as no crystal is the same as another, ever!
Science differentiates between twelve different types of ice in our universe. On Earth only one type of ice exists, the one we all know and it can float on water. Ice on Earth has many faces: Ice streams at the poles, glaciers in the high mountains, permafrost, frozen waterfalls, snow, hoarfrost, hail, Jack Frost on the windows…
The six corner foundation
The water molecules of an ice crystal always form a hexagonal grid made up of oxygen and hydrogen atoms. The oxygen atoms sit on the six corners and are connected via the hydrogen atoms. Every oxygen atom requires two hydrogen atoms, a formula we are very familiar with: H2O. From this grid we get the hexagon shape of the snow crystal.
A water molecule is tiny, very, very tiny! It takes 3.3 million H2O molecules to build a crystal chain of 1 millimeter.
Importantly, snowflakes are not frozen water drops! Sometimes raindrops freeze on their way to the ground but they are called Graupel (Little ice balls that look like styrofoam). They don’t have the artful, symmetrical shapes which are typical for snow crystals.
Snow crystals develop when water vapor condenses to ice, which happens high in the clouds.
The multifaceted shapes develop during the growth stage. The diameter of a frozen crystal grid is 0.0000045mm , or twelve water molecules in a chain.
The story of a snowflake begins with water vapor in the air. The evaporation of water from oceans, lakes and rivers, even the breathing of plants create this vapor. High up in the air this water vapor condenses on a dust particle that floats in the atmosphere. Incalculable numbers of droplets develop, each one containing at least one dust particle. A cloud contains a humongous amount of these droplets, that float in the air. The water content of a cloud varies between 0.05 and 0.3 g/m3. That doesn’t sound like much but a cloud that is 1000m tall and has a surface area of 1 km2 has an actual weight between 50 to 300 tonnes.
Whether Summer or Winter, it is always cold in a cloud, mostly below freezing. This is why we often experience hail from mid Summer storms. So why don’t the droplets just freeze and fall to the ground? To transform the water molecules into an ice grid it requires a jig. The dust particle is such a jig and gives the water molecule the “instructions” to freeze and guides the turbulent water molecules into an order. The formation of ice crystals can begin. This doesn’t happen at freezing point but requires a temperature of at least -10 C. This minute ice particle will grow into a snowflake as more and more water molecules connect and condense and freeze onto this starting ice particle.
For the construction of one snowflake we need an insane amount of water molecules, over 1000 trillion! To put this in perspective, a water molecule to a snowflake equals a ball with a diameter of 10m to our planet earth.
Mind boggling complexity
The snow crystal that is being developed in the cloud starts off with a six cornered prism. As it grows reasonably regular, branches sprout out of these corners. Life in a cloud is very turbulent and subject to constant temperature changes. The result is a very complex hexagonal ice crystal. The difference in snow crystals depends on humidity and temperature and turbulence within the cloud.
Sixth sense?
What synchronizes the growth of those six branches in a snow crystal? A complicated question with an easy answer: Absolutely nothing!
The six arms grow independently from each other but under the same environmental conditions. This often results in symmetry but doesn’t have to. Despite all the pictures of symmetrical snowflakes, most of them are irregular. With enough water vapor the prism crystal will grow and eventually fall out of the cloud. The water molecules that surround the crystal become less and less and have to connect from further away. This process is called diffusion. This diffusion through the air takes time and slows the growth. Our six cornered prism keeps wafting through the cloud while the tips of the six cornered flake stick out a little further than the rest. This raises the possibility that a diffusing water molecule docks on the tip, sticks out even further and creates docking points for more molecules. An ever growing circle of growth begins. This growth all depends on the temperature, humidity and air pressure .
The higher the pressure the more branches develop.
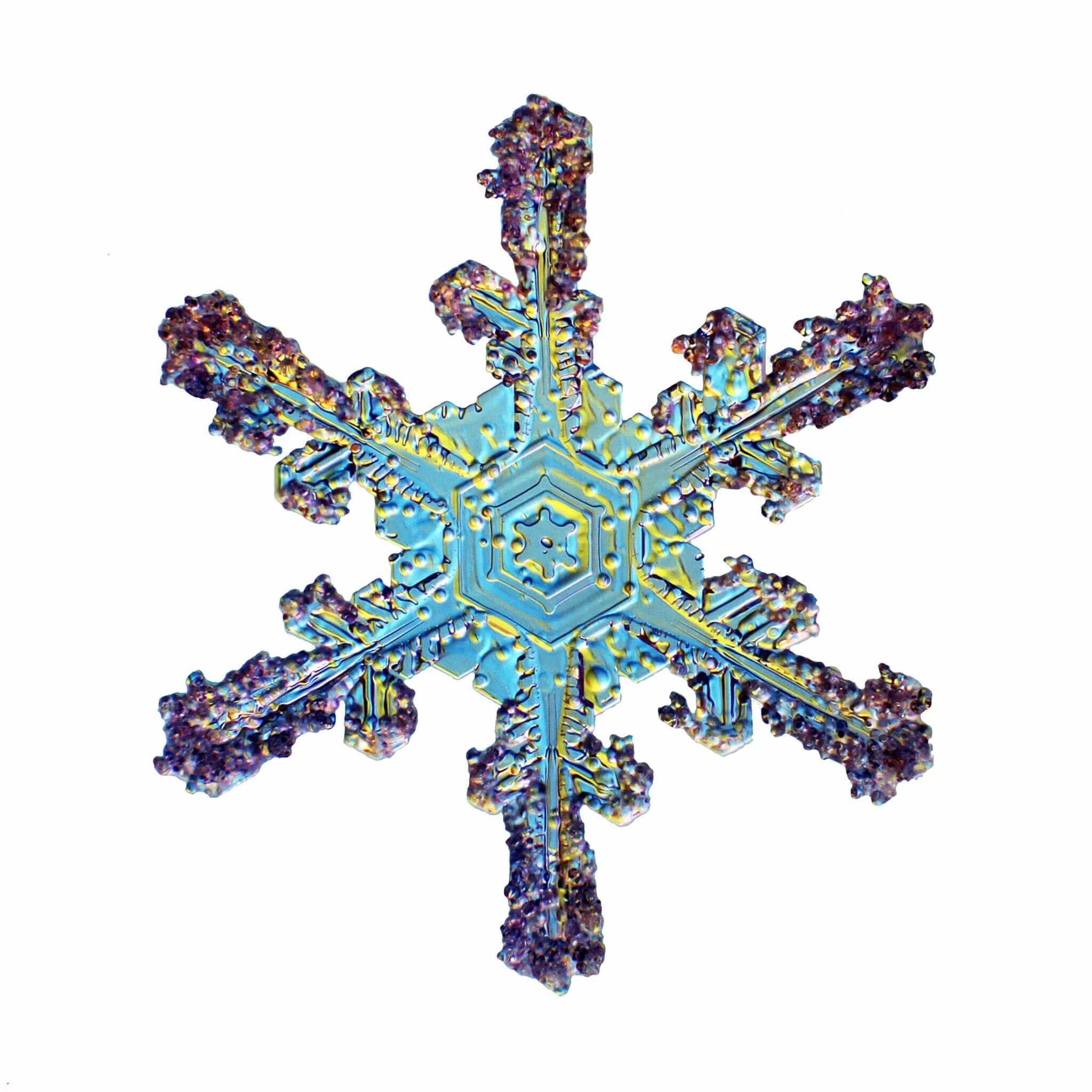
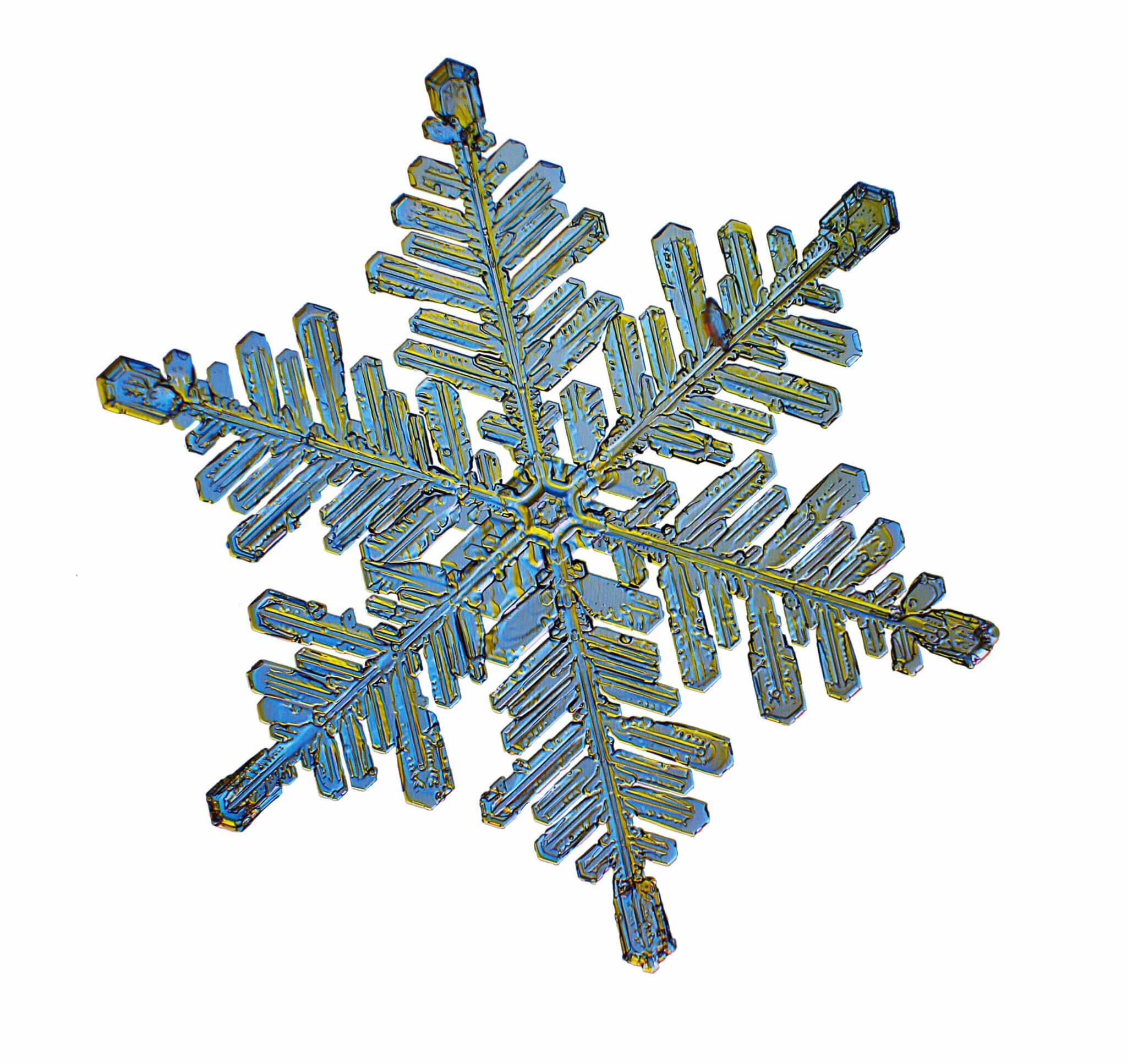
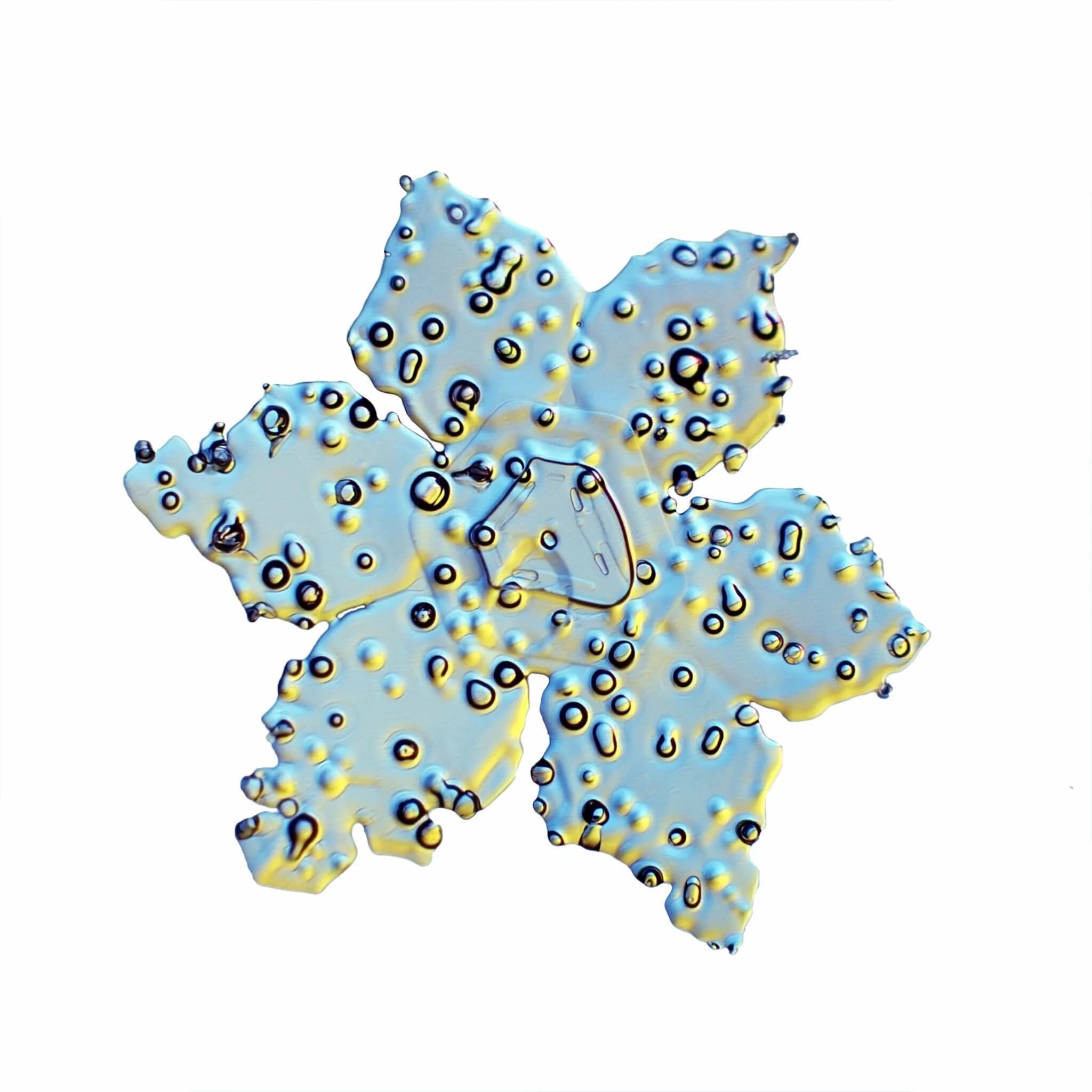
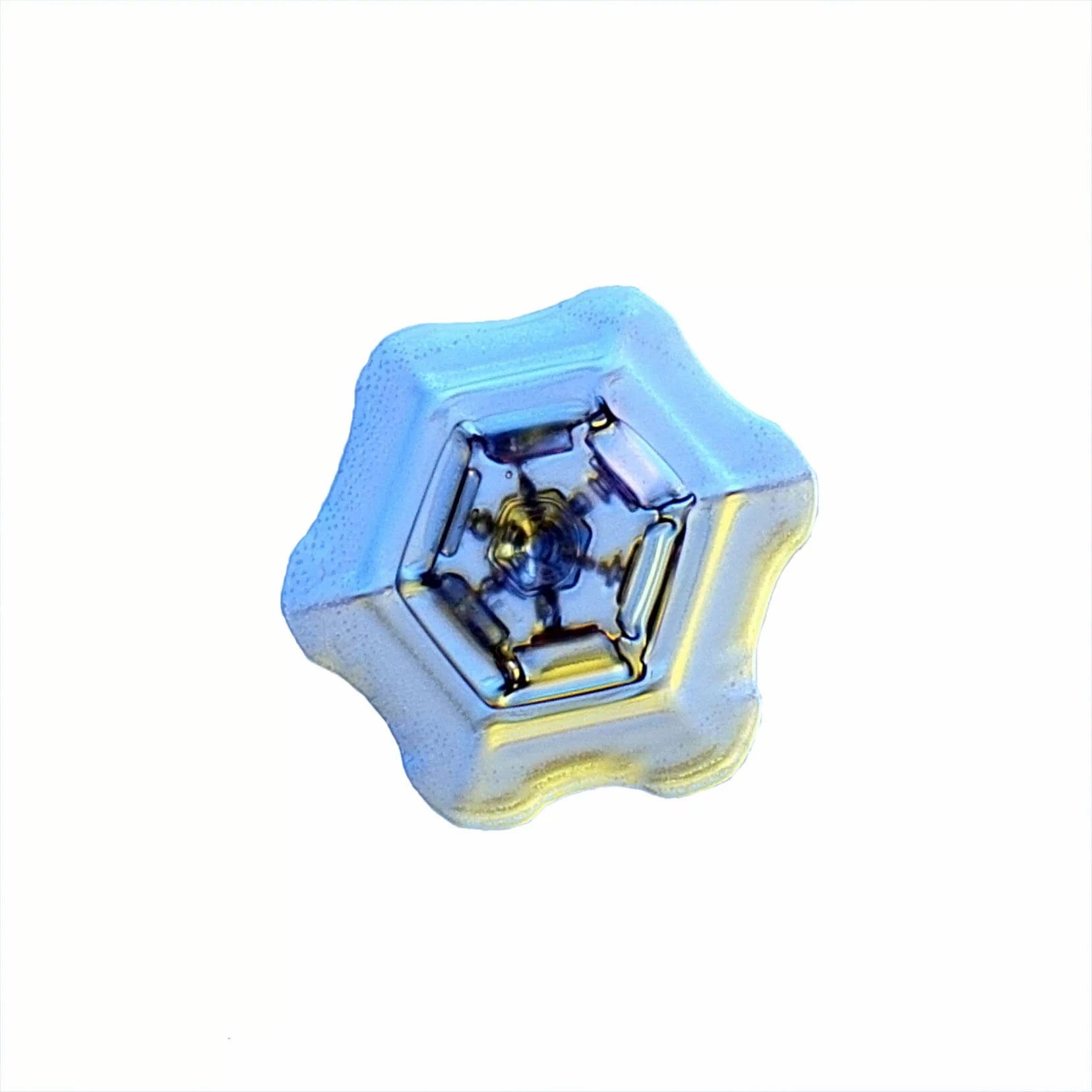
These Snow Crystals were photographed by Dr. Norbert Spam at an altitude of 1120m. The Snow Crystals are caught on a glass plate and positioned carefully into a special optical device. Here they are illuminated with 2 different colors to accentuate their structure.
All this has to happen quickly as they evaporate within a minute.
The magical shapes
Even when we create snow crystals in the lab we notice that the shapes are dependent on humidity and temperature. This behaviour we can combine in a morphological diagram.
We see between 0ºC and -3ºC the development of thin plates and the classic snowflake (Dendrites). Below -3ºC slender needles and columns grow. From -10ºC to -22ºC we see the reemergence of aesthetical stars , as well as plates and sector discs. Past -22ºC it snows only small prisms and plates.
Star Dendrites can be up to 5mm wide but are very flat with about 0.1mm thickness. They have six symmetrical branches with many random site branches. The largest of these develop around -15ºC and are the ones we love the most by giving us powder snow. The angle between the branches is always 60 Degrees or a multiple of that. The main arms are so amazingly symmetrical as they grow simultaneously from the corners of the Ice prism.
Sector Discs are noticeable due to their narrow burrs that divide the crystal in different sectors. Sometimes they appear on the branches of Dendrites or other discs. These crystals need special conditions for growth. If it is too humid we get star shaped branches, too dry and the discs grow slower and not as thin.
Columns and Needles are what fall out of the sky most often. They grow around -5ºC or when it is noticeably colder at -25ºC. With these crystals the bases grow faster than the sides and we get pencil-like hexagonal prisms whose insides are mostly conical and hollow.
With high humidity and -5ºC these columns are as thin as needles that can still be hollow.